Integrated generation and characterization of CAR-T cells

Chimeric antigen receptor (CAR) T-cell therapies have shown immense promise since the first FDA approval of a CAR-T cell therapy in 2017. However, CAR-T production remains a complex and sensitive process. Producing therapeutic CAR-T cells from a donor involves isolating T cells from a patient, activating these cells, introducing an engineered CAR construct and expanding the cells to a scale appropriate for therapeutic dosing.
Inconsistencies in raw materials – the patient samples – can lead to inconsistencies in product quality and clinical outcomes. To address this, rigorous quality control (QC) tests must be performed at each stage of the CAR-T production process. Key controlled characteristics include identity, purity and potency. Regardless of the characteristic, it should be measured and the data acted upon regularly to ensure product quality and patient safety. Robust processes that can reduce variability through the seamless integration of systems and improve the repeatability of testing with high-quality reagents are therefore critical.
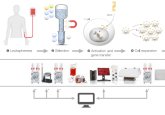
In this application note, learn more about a method and workflow for the generation and characterization of CAR-T cell products. T cells were isolated from healthy donors, expanded ex vivo and transduced with a second-generation (4-1BB and CD3ζ domains) anti-CD19 CAR lentivirus. Following CAR-T production, cell identity, purity and potency were characterized. This integrated and optimized workflow will enable consistent CAR-T production for use in therapeutic and other applications.